return to homepage The Electron Orbit
The standard model is full of gaping holes, but the hole of which I will remind you in this paper is among the largest. Despite its size and splendor, it has gone completely under the radar for decades now. It’s existence is like the existence of a hole in your bedroom floor: Um, Rosie, did you realize that you have a hole in your bedroom floor? No, I never really noticed it. It seems rather hard to ignore. It is six feet in diameter and is right next to your bed. Hm. I guess I just walk around it every morning on the way to the bathroom.
return to updates
in Quantum Mechanics)
by Miles Mathis
It is amazing the things we can look away from, when we really need to. The problem with the electron “orbit” is that the electron and proton have opposite charges, we are told. This causes an attraction, as we know. And yet the electron and proton only seem to attract each other up to a point. The electron is not attracted all the way into the proton itself, it is only attracted to the distance of some shell, near to the proton. This is fairly astonishing, or should be, and yet the standard model completely ignores it. It doesn’t even find it necessary to tell us why the electron doesn’t continue on in to collision. Wikipedia, for example, conspicuously avoids this question under all headings. In the few instances that QM or QED deigns to notice this problem, it tells us, mostly by implication, that the electron maintains its distance due to its orbital velocity.1 But this is no answer. Why should the electron, attracted to the proton, suddenly develop an orbital velocity? At what distance from the proton does it decide to start going sideways, and for what mechanical reason? We are led to assume, by the fudgy wording and theory of QED, that electrons must always just miss the proton, as if they always just happen to intersect the proton at a tangent, this tangent being the right orbital distance for the shell. But this is mystification in the extreme. Given particles that are rushing around with opposite charges, we would expect a large number of direct collisions. We would expect a fair number of direct collisions even without opposite charges, wouldn’t we? If these quantum particles were asteroids instead of electrons and protons, we would expect direct collisions, no matter their sizes. But if we add charge to the mix, we should expect a highly noticeable number of collisions.
Say you have an electron that just happens to be on an intersecting path with a proton. Is this impossible to imagine? No. Is it possible to imagine that electrons are never on an intersecting path with any proton? No. Now we add charge. The electron is not only on an intersecting path, it is attracted very strongly to the proton. Why does it not hit it?
The standard model has no answer. It just pretends it is not a problem, mostly by ignoring it. Texts never address it. It has been buried. To show this, let us do a search on the internet. Here is a “direct” answer from the University of Illinois physics department2:
Why doesn’t the electron get sucked into the nucleus since the nucleus is positive and the electron is negative?
-Matt (age 16)
That’s a really great question! The picture we often have of electrons as small objects circling a nucleus in well defined "orbits" is actually quite wrong. The positions of these electrons at any given time is not well known at all, however we CAN figure out the volume of space where we are likely to find a given electron. For example, the electron in a hydrogen atom likes to occupy a spherical volume surrounding the proton. If you think of the proton as a grain of salt, then the electron is about equally likely to be anywhere inside a ten foot radius sphere surrounding this grain, kind of like a cloud.
The weird thing about that cloud is that its spread in space is related to the spread of possible momenta (or velocities) of the electron. So here’s the key point, which we won’t pretend to explain here. The more squashed in the cloud gets, the more spread out the range of momenta has to get. That’s called Heisenberg’s uncertainty principle. Big momenta mean big kinetic energies. So the cloud can lower its potential energy by squishing in closer to the nucleus, but when it squishes in too far its kinetic energy goes up more than its potential energy goes down. So it settles at a happy medium, and that gives the cloud and thus the atom its size.
That’s a really terrible answer, even to give to a 16 year old! In fact, it is just gibberish. The problem is, it really does represent the full answer, the one given to graduate students when they insist upon one (which they rarely do). The full answer has more gibberish, but not more content or logic. QM tries to answer the question by making the electron a cloud or probability, but we must imagine that no matter how probabilistic the electron is, it still must have a negative charge. It cannot have a negative charge far away from the nucleus, acting like a particle, then approach the nucleus and begin acting like a cloud with a positive charge. All this talk of momentum and kinetic energy and HUP is just misdirection. No matter how you represent the kinetic energy or momentum of the electron, you cannot create a repulsion. The dispersion of momentum or kinetic energy into a cloud or probability cannot switch the charge or create a repulsion. And the HUP simply has nothing to say about switching charges or creating repulsions from attractions. This “physicist” should be ashamed to be saying such things, especially to the young.
The problem is, the standard model has no better answer than this, and they know it. Generally, when an adult has the temerity to ask this question, they are not treated with condescension and given this embarrassing answer. Instead, they are browbeaten. They are answered in this way (which I have boiled down from a thousand physics forums posts):
Idiot! Electrons are not spitballs. Go back to school and then get back to me, after you have your PhD.
This saves these PhDs from having to be bothered with basic theory. They can tell horrible lies to schoolchildren and growl at everyone else, and feel safe among their walls.
The truth is, using the wave function to represent electrons instead of representing them as discrete balls does nothing to answer this question. It is complete misdirection. I am not saying the wave function is mathematically incorrect. I am saying the wave function does nothing to explain repulsion. The wave function in no way gives the electron a positive charge, or turns off its negative charge. To do that we would have to change or stop the spin of the electron, and the wave function does not do this. In fact, the wave function was meant to represent some unknown motion or motions or amplitudes of the electron: its complex wave. This wave function does not change its character as the electron approaches the proton, it expresses this character. But for the proton to begin repulsing the electron, the electron would have to change its character. It would have to change its charge in some way.
The answer that includes momentum and HUP is especially dishonest, because it wants us to believe that a probability is somehow an exclusionary force of its own. Yes, the answer from Illinois above treats a probability as a force. Once you boil this argument down, it centers on the idea that making the electron a cloud is enough to explain why it doesn’t impact the proton. What you do is smear the electron out into a probability, then give the smear an edge. This edge is then given an exclusionary force, like a material bubble. The electron can’t impact the proton because the electron is now a big bubble, and the bubble bounces off the proton! Lovely.
The mainstream physicists always deflect these questions by screaming that the electron is not really in an “orbit.” It is in a probabilistic cloud. But a probabilistic cloud does not magically become repulsive just by becoming probabilistic. Probability math should not, and does not, switch the charge on the electron. The electron can have all the wave motions and functions and amplitudes and smearing it wants to have, but becoming a wave or a smear does not bypass this fundamental problem. This is because neither waves nor probabilities are automatically repulsive. There is no theoretical reason to believe that either waves or probabilities are physically exclusive. If they were, then protons and neutrons, which according to QED (see De Broglie, Pauli, Gell Mann, etc.) also have wave and probability characteristics, could never fit into the nucleus. Their bubbles would repulse each other, and the strong force would have to overcome not just E/M, but also probability repulsion.
This is also proved by the photoelectric effect and many other experiments. The photoelectric effect works both ways: if the photon acts like a particle, the electron must, also. Both the photon and the electron must not only have a discrete energy, they must have discrete positions, otherwise the data would not work like it does. Once again the standard model tries to fudge over this fact with probabilities, but a mechanical explanation requires that both the incoming and outgoing particles must have real position at impact. Energy transfer cannot take place mechanically between probabilities, since probabilities can only work mathematically. The photon must physically hit something, and you cannot hit a probability.
Modern physicists pretend that the HUP has something to say about this, but it doesn't. The HUP addresses the math of QM, not the material. The HUP is an operational rule, nothing more. In fact, even if we accept the HUP as applying to the electrons themselves, it fails to deflect all these questions. The HUP states that we cannot measure position and momentum at the same time, achieving accurate values for both. But it also states that if we don't care about momentum, we can measure position VERY accurately. Just look at the equation, ΔXΔP ≥ 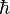 /2. If we make ΔP very large, we can make ΔX very small. In this case, the nasty question raises its head once more: why doesn't a very small ΔX impact the proton or nucleus?
/2. If we make ΔP very large, we can make ΔX very small. In this case, the nasty question raises its head once more: why doesn't a very small ΔX impact the proton or nucleus?
Quantum physicists can make up all the longwinded, jargon-heavy, illogical explanations they want, but there is a very simple explanation that requires no pettifogging, no faith, and no paradoxes.
It used to be that a person who could come up with a simpler, more transparent explanation was a better physicist. That is no longer the case. Now the person that can come up with the more convoluted, mysterious, wordy, and illogical explanation is the better physicist, since such an answer must seem more “profound.”
Of course, I would not bring up this problem and treat the opposition with such contempt if I did not have a better answer for it. Fortunately, I do, one that happens to be very simple and direct, as well as mechanical. I have shown in a series of papers that if we make the charge force mechanical, we must get rid of the messenger or virtual photon that is now said to mediate it. We must replace that virtual photon with a real photon, and give it mass equivalence. Moreover, we must make all force repulsive. There is simply no way to explain attraction mechanically, so we give up on attraction, at the foundational level. Underlying both electricity and magnetism, we have the charge field, or what I now call the foundational E/M field. Although electricity may be either positive or negative, the foundational E/M field is always positive. It is always repulsive. This means that all protons and electrons are emitting real photons, and that all protons and electrons are repulsing all other protons and electrons, via simple bombardment. Attraction is explained by noticing that protons repulse electrons much less than they repulse other protons. In this way, the attraction is a relative attraction. Relative to the speed of repulsion of protons with one another, electron appear to move backwards. If protons are defined as the baseline, then electrons are negative to this baseline.
Classically, this can be explained by the size difference alone. Due only to surface area considerations, electrons are able to dodge much of the emission of protons and nuclei, and so they seem to swim upstream.
If you want to think of protons and electrons as smears instead of particles, be my guest: it doesn’t change my analysis at all. Larger smears repel smaller smears less than they repel each other. Smears have size just like particles, and electron smears must have smaller or less dense smears than protons. Or, probability smears of electrons must have less flux, or whatever. However you want to define or imagine the electron, the electron must have more space in or around its probability smear, which means my analysis must hold in any possible field. This being true, I think it is much preferable, from a theoretical viewpoint, to talk of discrete particles. Talking of smears adds nothing to the fundamental theory, and, in fact, often throws a blanket over it.
This explains our current problem in a very direct manner, since the orbital distance or shell or level that the electron ultimately reaches is determined by the distance at which the electron is no longer able to dodge the emission of the proton. If we think of the electron and proton as spheres, it makes this very easy to see (we can think of the spheres as probability clouds rather than particles, if we like, but it does not change the mechanics in any important way). The proton is emitting at a constant rate, we assume. But due to spherical considerations, the emission field must dissipate with greater distance from the center. Which is the same as saying that it gets denser the closer you get to the proton. The electron simply continues to fall nearer the proton, until the field density of emitted photons gets great enough to stop it. At this point, a level of equilibrium is reached. The proton has always been repulsing the electron, but now the electron gets close enough that the proton can stop it from coming nearer. At greater distances, the field density of photons was not enough to stop the electron, but now it is great enough. It is that simple.
Think of it this way, if you like. Let us say you live inside a proton, and you have a little window you can look out of. You are very private, so you have an ingenious intruder system. You have guns mounted all around your spherical “house”, but instead of firing bullets, they fire basketballs. All your neighbors are protons, and you have found that you can keep these drifting neighbors away using these basketballs. By long experience, you have found that using a given rate of fire, these neighboring protons never get closer to you than 100 feet. You have also found that at 100 feet, these neighboring protons have an apparent size of one foot. At that distance, they can’t really tell what you are doing and you can’t tell what they are doing, so you are satisfied. Everything is great until an electron moves into the neighborhood. The problem is, he is a lot smaller and he can navigate the gaps between basketballs. He can only move in a straight line, so many times he gets hit and you keep him away. But over time, by trying again and again, he is able to get quite near. After long years of this annoyance, you find from your records that this electron is able to get 10 feet from your house, but no nearer. Here is the question: how big is the electron’s apparent size at 10 feet?
You don’t have to ask Marilyn; I will tell you. The answer is: one foot. The electron can defy the field until he reaches the point of optical equivalence to the neighboring protons. At this point the pressure of basketballs on him at ten feet is equal to that on the neighboring protons at 100 feet. Or, to say it in another way, if two basketballs per second hit the protons at 100 feet, two basketballs per second will hit the electron at 10 feet.3
Now, the point of this story was not to imply that the proton is 100 times bigger than the electron or to imply that the proton is a simple sphere, any more than it was to imply that little people live inside protons. The point of the story is to show that there is a logical variant to the standard model explanation. We do not have to believe in opposite charges causing attractions or repulsions. We do not have to believe in messenger photons that are capable of “telling” quanta whether they should move nearer or farther away. We can propose a simple bombarding field like this and use it to explain protons repelling and also to explain electrons coming close to the protons. One of the great benefits of this new theory (and there are many many others) is that it explains all at once why the electron does not fall into the proton. It does not collide because it was never attracted to the proton or the nucleus in the first place. Its distance of exclusion is simply much less, based on its size.
Also notice that we can throw this theoretical switch without affecting most of the math of QM and QED. Yes, it will require some fundamental changes, but the bulk of the mathematical content of the wave equations is unaffected. More than anything, this is a shoring up of the foundations, not a critique of the math. Above all, throwing this switch opens up the road to unification, as I have shown elsewhere. Quantum physicists can proudly keep much of their edifice; but now it is possible to unify that edifice with gravity, in a transparent manner, without the need of strings or other esoterica. It is also now possible to answer the simple questions of high school students without telling embarrassing fibs.
1http://answers.yahoo.com/question/index?qid=20060620215656AAW82ZS
2http://van.physics.uiuc.edu/qa/listing.php?id=1226
3This short answer assumes that the electron and proton weigh the same, and so “feel” the same force or pressure. Of course they don’t, so the answer is incomplete. For optical equivalence to work, we would have to include the gravity field here, as well as the foundational E/M field, and I haven‘t wanted to get into that. Gravity is present at the quantum level, so my answer is strictly correct. Once we include gravity, all we have to do is assume that the proton and electron have the same density. In which case the falling off of gravity exactly offsets the difference in mass. The repulsive force is 100 times less, per unit area; but the “attractive” force of gravity is also 100 times less, so they cancel.
If this paper was useful to you in any way, please consider donating a dollar (or more) to the SAVE THE ARTISTS FOUNDATION. This will allow me to continue writing these "unpublishable" things. Don't be confused by paying Melisa Smith--that is just one of my many noms de plume. If you are a Paypal user, there is no fee; so it might be worth your while to become one. Otherwise they will rob us 33 cents for each transaction.